Veselin Mitkov
University of Mining and Geology “St. Ivan Rilski”
https://doi.org/10.53656/nat2025-4.05
Abstract. An experimental equipment is constructed for testing the effect of gas-hydrogen mixture at different % hydrogen and methane contents (5%, 15%, 25%) on the steel tubing used for production and injection of gas wells for underground gas-hydrogen storage. The presented publication consists of 5 modules: Introduction to the problem that is at hand, theoretical look over the presented issue, the experimental equipment is shown and thoroughly explained, the steps necessary for conducting the experiment are shown, results and summarizing them. The results obtained from the conducted experiment show the potential of production and injection gas-hydrogen mixture with steel tubing for underground storage in underground gas storage facilities.
Keywords: hydrogen, steel pipeline, well tubing, hydrogen embrittlement
Introduction
The continuous growth of the world population and economic activities has led to a significant increase in energy demand over the last century. This demand has been met mainly through the consumption of carbon-based fossil fuels, including coal, oil, and natural gas. However, the burning of fossil fuels releases greenhouse gases into the atmosphere, causing global warming and abnormal weather events that pose a serious threat to modern society. To mitigate these climate impacts, an urgent transition to carbon-neutral energy sources is needed. Among the potential alternatives, hydrogen is emerging as a promising energy carrier capable of supporting global decarbonization.
Hydrogen also offers economic and technical advantages. As a fuel, it offers a higher energy content per unit of mass compared to fossil fuels. This makes hydrogen an attractive candidate for replacing conventional energy sources in both industry and transport. However, the creation of a hydrogen-based energy system depends largely on cost-effective and safe methods of operation.
Pipeline transport is currently the most technologically advanced and large-scale approach to hydrogen delivery. Operating pipelines at elevated pressures improves transport efficiency but leads to additional energy requirements and costs. In the United States alone, over 1,600 miles of dedicated hydrogen pipelines have already been built. This is in addition to over 300,000 miles of natural gas pipelines that are expected to be converted to transport natural gas mixed with hydrogen in the short term, and ultimately pure hydrogen in the long term (Dincer & Acar, 2015; Li et al., 2022).
The movement of the extracted and injected fluid (gas-hydrogen mixture) from the bottomhole to the wellhead is carried out through a column of pump-compressor pipes (PCP) or tubing that are lowered into the production column. The purpose of the tubing is to ensure the movement of the extracted or injected fluid. The tubing column also protects the production column from abrasive and corrosive effects between the gas-hydrogen mixture and the production column. The tubing column facilitates repair work and is also necessary for stopping the well in emergency situations. The tubing is used to ensure a suitable technological regime for well operation (Gerov, 2019).
Most gas pipelines are constructed from steel pipes, which are produced economically in large quantities. Hydrogen pipelines have historically relied on medium-strength steels, such as American Petroleum Institute (API) 5L X42, X46, and X52, which can operate at pressures up to 13 MPa. With the growth of industrial demand for pipelines capable of transporting hydrogen at higher pressures, research has focused on higher-quality steels such as API 5CT API 5L X60, X70, and X80. Despite their higher strength, these high-quality steels are more susceptible to hydrogen embrittlement (HE), a phenomenon in which the presence of hydrogen causes the steel to become brittle. Despite their higher strength, these high-quality steels are more susceptible to hydrogen embrittlement (HE), a phenomenon in which the presence of hydrogen reduces the strength and/or ductility of metals, leading to emergency conditions. Given that hydrogen is highly flammable in the presence of oxygen, any leak in hydrogen transport pipelines can lead to catastrophic accidents (Li et al., 2022).
Four conditions are usually necessary for hydrogen embrittlement to occur in steels:
- Hydrogen that can diffuse into the microstructure.
- The presence of microstructural features in the steel pipeline that are susceptible to HE, such as cracks.
- Applied or residual stresses.
- Sufficient time for hydrogen atoms to diffuse to these susceptible areas.
Long-term operation of metal pipelines transporting hydrogen under high pressure can easily satisfy all these conditions. In addition, additional operating variables (temperature and pressure), such as local stress concentration and cyclic loading, can exacerbate hydrogen embrittlement under operating conditions (Abe et al., 2019; Momirlan & Veziroglu, 2002).
For these reasons, it is crucial to investigate and study the mechanisms of hydrogen embrittlement in steel pipelines before their large-scale implementation in operation. This publication analyzes the interactions of hydrogen with steel surfaces, the main factors affecting susceptibility to hydrogen embrittlement, and existing methods for studying hydrogen embrittlement (Hawkins & Joffe, 2006).
This publication presents a developed test bench for rapid determination of the influence of gas-hydrogen mixtures on the tubing in the process of injection and extraction. The developed research test bench consists of four main modules:
- Bottle with standard gas-hydrogen mixture; regulator; flexible connection for connecting the regulator to the second module
- Set of tubing with diameters 2′ 7/8 and 4′ 1/2 and with lengths 42 cm
- Measuring manometers with a range of 25 bar and high-pressure valves
- Equipment for measuring gas-hydrogen mixture leaks and equipment for determining the technical condition of the tested tubing (flaw detection equipment).
Theoretical
Physical and chemical properties
Hydrogen (symbol H, atomic number 1) is the lightest element and is colorless, odorless, and tasteless. Although it is placed in group 1 of the periodic table with the alkali metals, hydrogen is not a metal. Under normal conditions, it behaves like a non-metal and easily forms a single covalent bond with most non-metallic elements, leading to the formation of compounds such as water and most organic molecules. Only under extremely high pressure does hydrogen exhibit metallic properties (Table 1).
Table 1. Physical and chemical properties of hydrogen
| Chemical Symbol | H2 |
| Phases | Gaseous, Liquid, Slush, Solid, Metallic, and Plasma |
| Molecular weight | 2.016 g.mol-1 |
| H-H bond dissociation energy | 435.7 kJ.mol-1 |
| Ground state energy level of the electron in an atom | -13.6 eV |
| Specific volume of gas at 70 oF (21.1 oC) and 1 atm | 11.99 m3.kg-1 |
| Autoignition temperature | 570 oC |
| Boiling Point at 1.01 bar | -252.8 oC |
| Melting Point at 1.01 bar | -259.2 oC |
| Relative density (air=1) 1atm | 0.08989 g.ml-1 |
| Gaseous Energy Content | 120 MJ.kg-1 |
| Liquid Hydrogen Energy Content | 8 MJ.L-1 |
| Critical Temperature | -239.9 oC |
| Critical Pressure | 12.96 bar, abs |
| Critical density | 30.12 kg.m-3 |
| Triple point | -259.3 oC at 0.07 bar, abs |
| Flammable limits in the air (by volume) | 4.0-75.0% |
| Enthalpy of Combustion | -286 kJ.mol-1 |
| Latent heat of fusion at the triple point | 58.09 kJ.kg-1 |
| Latent heat of vaporization at boiling point | 446.0 kJ.kg-1 |
| Cp | 14.34 kJ.kg-1 oC |
| Cv | 10.12 kJ.kg-1 oC |
| Ratio of specific heats | 1.42 |
| CAS No. | 133-74-0 |
| UN No. | 1049 |
| ERG No. | 115 |
Hydrogen is the lightest element, but it provides the highest energy per unit mass of all known fuels. However, its volumetric energy density is very low. To overcome this limitation, gaseous hydrogen is typically compressed to pressures between 200 and 700 bar. Alternative approaches, such as cryogenic compression and material-based storage, are also being developed to increase the efficiency of hydrogen storage (Messaoudani et al., 2016).
Hydrogen embrittlement is the loss of plasticity and strength in metals caused by the introduction of hydrogen atoms into the lattice, usually at temperatures below ~150 °C. Hydrogen can be introduced during high-temperature operations (casting, welding, heat treatment) or electrochemical processes (corrosion, electroplating, cathodic protection). Once absorbed, it spreads to the grain boundaries, where it forms high-pressure bubbles or brittle hydrides (in metals such as titanium), leading to intergranular cracking. This changes the fracture mode of the metal from ductile to brittle. In hydrogen storage tanks, types I–III (metal or metal-coated) are vulnerable throughout their structure, while type IV (composite with polymer coating) are vulnerable only in the metal part where the valve is located (Ghadiani et al, 2024; Subedi & Thapa, 2023).
Experimental
Fig. 1 shows the research equipment developed for rapid determination of the effect of gas-hydrogen mixtures on the tubing.
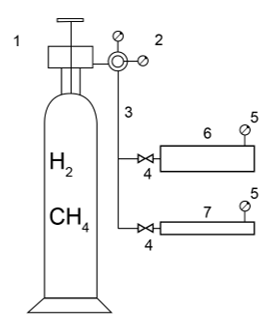
Figure 1. Diagram of the developed equipment
1 – Bottle with gas-hydrogen mixture (5, 15, 25% hydrogen content); 2 – Pressure regulator; 3 – Soft coupling; 4 – High-pressure valve; 5 – High-pressure manometer; 6 – Tubing 4‘1/2; 7 – Tubing 2‘7/8
Table 2 shows the characteristics of the gas-hydrogen mixture bottles. The characteristics include working pressure, temperature, bottle volume, and quantity. Table 3 shows the certificate for the gas-hydrogen mixture in cylinders.
Table 2. Characteristics of bottles containing a gas-hydrogen mixture
| Bottle № | Pressure at 15 oC | Min. residual pressure | Volume | Temeprature of storage | Quantity |
| 13325 | 150 bar | 5 bar | 10 L | 5 °C – 30 °C | 1,5 m-3 |
| 13481 | 150 bar | 5 bar | 10 L | 5 °C – 30 °C | 1,5 m-3 |
| 02440 | 150 bar | 5 bar | 10 L | 5 °C – 30 °C | 1,5 m-3 |
Table 3. Certificate of the gas-hydrogen mixture
| Bottle № | Order number | Type of test | Unit of measurement | Test method | Set value | Measured value | Extended uncertainty |
| 13325 | 1 | Hydrogen (H2) | Vol.% | BDS EN ISO 6143:2006 | 5,00 | 5,00 | 0,14 |
| 2 | Methane (CH4) | 95,00 | 95,00 | 2,73 | |||
| 13481 | 1 | Hydrogen (H2) | 15,00 | 15,02 | 0,43 | ||
| 2 | Methane (CH4) | 85,00 | 84,98 | 2,44 | |||
| 02440 | 1 | Hydrogen (H2) | 25,00 | 24,94 | 0,71 | ||
| 2 | Methane (CH4) | 75,00 | 75,06 | 2,15 |
The measured values are based on volumetric parts at 0 °C and 1013 mbarTest conditions: 24.0 °C ±0.6 °C
Figure 2 shows a regulator for controlling the pressure of the gas-hydrogen mixture in the test process. Figure 3 shows a high-pressure valve. Figure 4 shows a measuring pressure gauge used in the equipment. Figure 5 shows the ultrasonic thickness gauge used for measuring the thickness of metal pipes. Figure 6 shows a gas analyzer for determining leaks of the gas-hydrogen mixture during the test.

Figure 2. Regulator for gas-hydrogen mixture – 825D-15-H
The selected regulator for gas-hydrogen mixture is made of forged brass body, stainless steel diaphragm – without internal contamination. The maximum inlet pressure of the regulator is 230 [bar]. A large diaphragm with a diameter of 70 mm is used to stabilize the working pressure. The flow rate of the regulator is [m3.h-1]. The maximum outlet pressure is 15 [bar]. The connection ports are: inlet port W21.80×1/14“LH and outlet port G3/8LH.

Figure 3. High pressure valve – GEMELS GE2
Type: ball valve 2 way, Sizes: from DN6 up to DN25, Ends: BSP-NPT-SAE-DIN, Pressure: up to PN500, Temp range: from -30°C to +100°C.
Certifications:
– CE ATEX – The CE certification ATEX (ATmosphere EXplosive) regulates equipment used in areas with an explosive atmosphere in the EU. DNV
– The DNV certificate (Det Norske Veritas body) regulates products and equipment conformity to the maritime regulations and requirements. EAC
– The EAC certificates and declarations (EurAsian Conformity) demonstrates that products conform to all technical regulations of the Eurasian Customs Union, particularly referring to pressure equipment, those used in areas with an explosive atmosphere and machines. UKCA
– The UKCA Ex certification (UK Conformity Assessed EXplosive atmosphere) regulates equipment used in areas with an explosive atmosphere in the United Kingdom. The UKCA PER certification (UK Conformity Assessed Pressure Equipment Regulation) regulates the design, manufacture, equipment and conformity assessment of pressure equipment ensuring a high level of safety in the United Kingdom1.
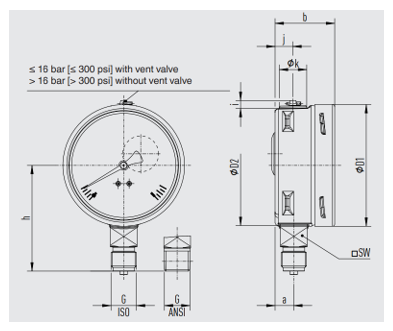
Figure 4. Bourdon tube pressure gauge, Wika Model 233.50
This high-quality Bourdon tube pressure gauge has been designed especially for the process industry. The use of high-quality stainless steel materials and the robust design are geared to applications in the chemical and process engineering industries. Thus the instrument is suitable for liquid and gaseous media, also in aggressive environments. WIKA manufactures and qualifies the pressure gauge in accordance with the standards EN 837-1 and ASME B40.100. As a safety function, this instrument has a blow-out device with blow-out plug on the back of the case. In the event of a failure, overpressure can escape there2.

Figure 5. Olympus 45MG
The 45MG is an advanced ultrasonic thickness gauge packed with standard measurement features and software options. This unique instrument is compatible with the complete range of Evident dual element and single element thickness gauge transducers, making this innovative instrument an all-in-one solution for virtually every thickness gauge application. In its basic configuration the 45MG is a simple and straightforward gauge that requires minimal operator training to tackle most common thickness gauging applications. With additional optional software options and transducers however, the 45MG can become significantly more advanced and take on applications well beyond that of a typical entry-level gauge. Furthermore, most options are available individually at the time of purchase or can be added in the future as your needs change3.
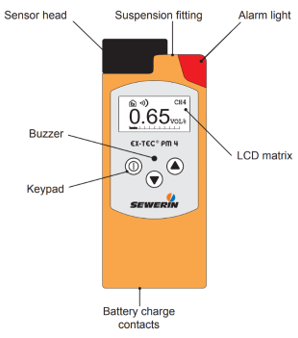
Figure 6. Sewerin EX-TEC PM 4
The EX-TEC PM 4 is an electronic handheld device for the detection and measurement of gas concentrations. Equipped with three sensors, it can be used for the ppm range, the % vol. Range and the LEL range. Intended use: According to DVGW Note G 465-4 the device can be used for the following purposes:
– Testing in houses/buildings, e.g. measuring minute gas concentrations in buildings and locating the origin of the gas.
– Testing in enclosed spaces, e.g. measuring the gas concentration in enclosed spaces or shafts with an increased potential of gas dispersal.
– Warning against explosive gas concentrations, e.g. for monitoring work areas whilst carrying out work to gas pipes or gas systems.
– Measurement of gas concentrations, e.g. when decommissioning gas systems.
Тhe EX-TEC PM 4 can detect the following gases:
Methane (CH4), Propane (C3H8), Butane (C4H10), Hexane (C6H14), Nonane (C9H20), Kerosene (JetFuel), Hydrogen (H2), Acetylene (C2H2)
Table 4 shows the characteristics of the tubing. The characteristics include outer diameter, weight per linear meter, wall thickness, steel grade, collapse resistance, internal yield pressure, and yield strength4.
Table 4. Characteristics of the tubing
| Outside Diameter | Weight | Wall Thickness | Grade | Pipe Body | ||||||||
| Collapse Resistance | Internal Yield Pressure | Yield Strenght | ||||||||||
| inch | mm | ppf | кg.m-1 | inch | mm | psi | MPa | psi | MPa | lb | kN | |
| 2 7/8 | 73.02 | 8.60 | 12.80 | 0.217 | 5.51 | P110 | 21040 | 145.0 | 20620 | 142.1 | 273200 | 1215.2 |
| 4 1/2 | 114.3 | 13.50 | 20.09 | 0.271 | 6.88 | P110 | 10690 | 73.7 | 12410 | 85.5 | 422000 | 1877 |
Sequence of conducting the experiment
After constructing the research equipment shown in Figure 1, the following research activities were carried out.
- Each of the tubing is examined with the Olympus MG45 ultrasonic instrument at three separate points along the length of the pipe to accurately determine the wall thickness and identify any defects. As a result of the examination, no such defects were found in any of the six tubing pipes.
- Testing the tubing for leaks. For this purpose, they are hydraulically tested for 30 days. Each pipe is pressurized with natural gas at a pressure of 20 bar. All the tubing is airtight, as a result of the hydraulic test, as no abnormal pressure drop was recorded over a period of 30 days (Boyadjiev & Georgiev, 2020).
- Each of the tubing is examined with the Olympus MG45 ultrasonic instrument at three separate points along the length of the pipe to accurately determine the wall thickness and identify any defects. As a result of the examination, no such defects were found in any of the six tubing pipes.
- The natural gas is released from all the tubing and then they are pressurized with a gas-hydrogen mixture with a hydrogen to methane concentration of 5, 15, and 25%.
- The following activities are carried out over a period of 30 days:
– Visual inspection of the tubing pipes for corrosion or breaches in the integrity of the equipment.
– Monitoring and recording the pressure in the tubing with a pressure gauge shown in Fig. 4.
– Measuring the wall thickness of the tubing with Olympus 45MG shown in
Fig. 5.
– Check for gas-hydrogen mixture leakage from threaded connections, welded joints, and the valve using a gas analyzer shown in Fig. 6.
Result and discussion
The result from the carried out experiment are shown in Table 5 and in Table 6.
Table 5. Results from testing the thickness of the tubing.
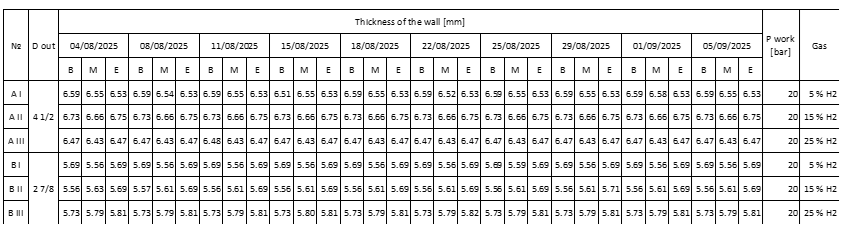
Note: B stands for Beginning, M stands for Middle and E stands for End. The three measurements are made 10 cm from one another.
Table 6. Pressure inside the tubing during the experiment.
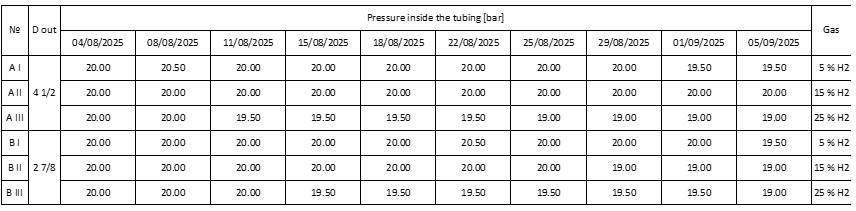
From the results shown in Table 5 from the carried out experiment, it can be said that the experiment has provided positive results as it’s shown from the data, that the thickness of the walls of the tubing remains a constant with small deviations that are caused from the Olympus 45MG. The recorded deviation is between 0.01 mm and 0.05 mm. For this reason, we can state that the gas-hydrogen mixture of 5, 15, 25% at 20 [bar] of work pressure didn’t affect the tubing column in the 30 days that the experiment was carried out.
From the results shown in Table 6, we can see the pressure inside the tubing during the 30 days that the experiment was carried out. The results show that in some of the tubing there is loss in pressure which is in a normal margin. During the experiment, the tubing was tested for leaks using the Sewerin EX-TEC PM 4, the gas detector signalized that there is a leak of gas-hydrogen mixture from the outlet of the GEMELS GE2 valves. The gas detector didn’t show any signs of leakage of gas-hydrogen mixture on the welded joints of the tubing, nor the threaded joints on the pressure gauge nor the inlet of the GEMELS GE2 valves. With this data it can be said that during the experiment, the tubing remained airtight (hermetic) and no pressure was lost due to the tubing made from P110 steel.
Conclusions
As a result of the developed research equipment for determining the influence of the gas-hydrogen mixture on the column of pump-compressor pipes for the research period, it was established that:
- The developed equipment is airtight (hermetic).
- A gas-hydrogen mixture with a hydrogen concentration of 5, 15, 25% has no effect on the tubing column made of P110 steel.
- The tested tubing with a diameter of 2’7/8 and 4’1/2 made of P110 steel can be used in the process of production and injection in underground gas storage facilities of a gas-hydrogen mixture with a hydrogen concentration of 5, 15, 25%.
Acknowledgements
This research is supported by the Bulgarian Ministry of Education and Science under the National Program “Young Scientists and Postdoctoral Students – 2” (Stage II 2024 – 2025).
Authors’ contributions
V.M. conceived of the presented idea, designed and built the experimental equipment, did all the necessary measurements and thoroughly took a record of them, wrote the manuscript.
NOTES
- https://www.gemels.com
- https://www.wika.com/en-en/homepage.WIKA
- https://ims.evidentscientific.com/en/
- https://www.voestalpine.com/group/en/
REFERENCES
Abe, J. O., Popoola, A., Ajenifuja, E., Popoola, O. (2019). Hydrogen energy, economy and storage: review and recommendation. Hydrogen energy, economy and storage: review and recommendation, 44(29), 15072 – 15086. https://doi.org/10.1016/j.ijhydene.2019.04.068.
Boyadjiev, M. & Georgiev, L. (2020). Running of gas infrastructure. University of Mining and Geology “St. Ivan Rilski”. [in Bulgarian]
Dincer, I., & Acar, C. (2015). Review and evaluation of hydrogen production methods for better sustainability. International Journal of Hydrogen Energy, 40(34), 11094 – 11111. https://doi.org/10.1016/j.ijhydene.2014.12.035
Gerov, L. (2019). Natural gas production and storage. UMG “St. Ivan Rilski”. [in Bulgarian].
Ghadiani, H., Farhat, Z., Alam, T. & Islam, M. A. (2024). Assessing Hydrogen Embrittlement in Pipeline Steels for Natural Gas-Hydrogen Blends: Implications for Existing Infrastructure. Solids, 5(3), 375 – 393. https://doi.org/10.3390/solids5030025
Hawkins, S. & Joffe, D. (2006). Technological Characterisation of Hydrogen Storage and Distribution Technologies. Policy Studies Institute.
Li, H., Niu, R., Li, W., Lu, H., Cairney, J., & Chen, Y-S. (2022). Hydrogen in pipeline steels: Recent advances in characterization and embrittlement mitigation. Journal of Natural Gas Science and Engineering, 105, 104709. https://doi.org/10.1016/j.jngse.2022.104709.
Messaoudani, Z. I., Rigas, F., Hamid, M.D.B., Hassan, C.R.C. (2016). Hazards, safety and knowledge gaps on hydrogen transmission via natural gas grid: a critical review. International Journal of Hydrogen Energy, 41(39), 17511 – 17525. https://doi.org/10.1016/j.ijhydene.2016.07.171.
Momirlan, M. & Veziroglu, T. (2002). Current status of hydrogen energy. Renewable and Sustainable Energy Reviews, 6(1 – 2), 141 – 179. https://doi.org/10.1016/S1364-0321(02)00004-7.
Subedi, A. & Thapa, B. S. (2023). Compendium of Fundamentals of Hydrogen Technology. Kathmandu University.
Veselin Mitkov, PhD Student
University of Mining and Geology “St. Ivan Rilski”
“Prof. Boyan Kamenov” Str.
Sofia, 1700, Bulgaria
E-mail: veselin.mitkov@mgu.bg
>> Download the article as a PDF file <<



